Have you ever wondered what truly happens when something turns from a liquid into a solid, especially when we talk about that special temperature in Celsius? It's a rather common thing we see every day, like when water turns into ice cubes for your drink, but the actual process is quite fascinating, you know. We often just accept that cold things freeze, but there's a whole lot going on at a tiny, invisible level that makes it all happen. This change, from a flowing liquid to a firm solid, is something that touches many parts of our lives, from keeping our food fresh to understanding the weather outside.
So, we're going to take a closer look at this cool transformation, focusing on the freezing point in Celsius, which is pretty much the standard for scientific measurements around the globe. We'll explore what it means for something to lose enough warmth to become solid and how this simple idea influences so much around us. It's not just about a number on a thermometer; it's about the very nature of matter changing its form, which is quite interesting, isn't that right?
This process of freezing, you see, isn't just a simple switch being flipped. It involves molecules slowing down and getting closer, creating a new, more organized arrangement. We'll also touch on what makes this process faster or slower, and what might even change the temperature at which something freezes. It’s a concept that is, basically, fundamental to many aspects of our world, from keeping your favorite frozen treats just right to predicting what might happen on a chilly winter morning, too it's almost a kind of magic, really.
- Winslow Cool Breeze On The Underground Download
- Latham Vpn
- 2026 Libra Horoscope
- Michelle Mclean Attorney
- If You Were Born In 1965 How Old Are You In 2023
Table of Contents
- What Exactly is Freezing and the Freezing Point in Celsius?
- How Does Temperature Affect This Process at the Freezing Point in Celsius?
- What Things Can Influence the Freezing Process and the Freezing Point in Celsius?
- Why is Rapid Freezing Often Better for Food When Considering the Freezing Point in Celsius?
- Freezing Rain - A Unique Weather Event Related to the Freezing Point in Celsius
- Understanding Water's Freezing Point in Celsius and Other Scales
What Exactly is Freezing and the Freezing Point in Celsius?
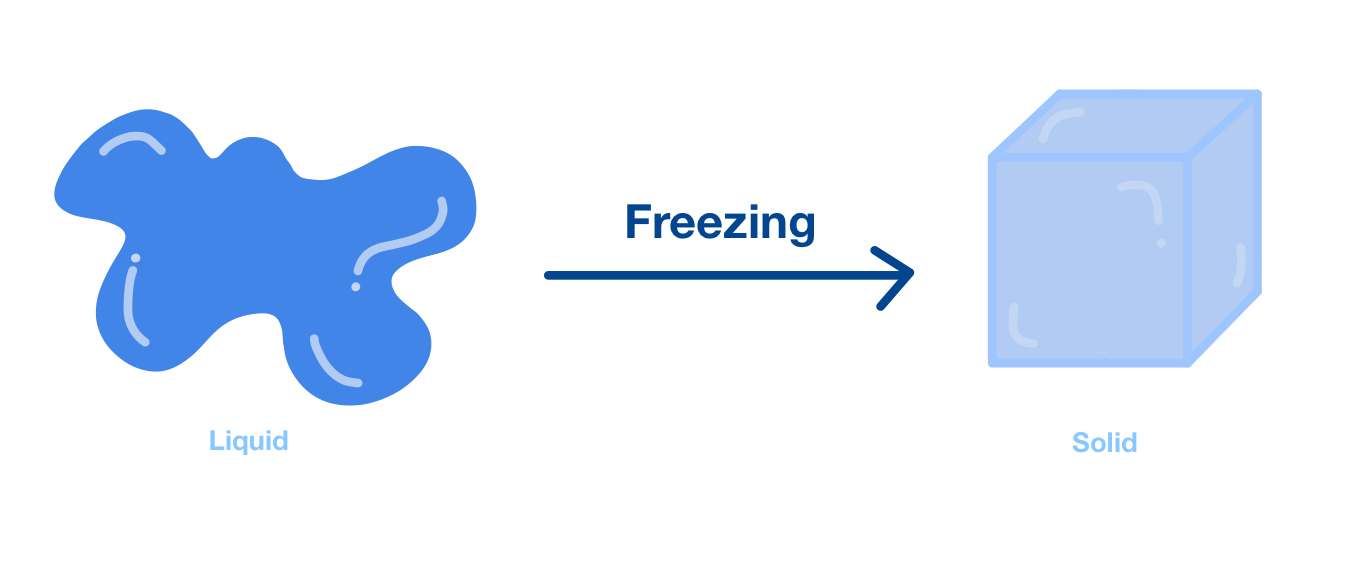
Freezing, at its heart, is a rather simple idea: it’s when a liquid substance turns into a solid one. This happens because the liquid gives away enough of its heat energy, basically getting cold enough to settle down into a firm shape. It’s a very common kind of shift that matter goes through, one of those main ways substances change their physical form, you know. Think about an ice cube: it starts as water, a liquid, but once it gets cold enough, it becomes a solid block of ice. This transformation happens at a very particular temperature, which we call the freezing point. For plain water, this specific temperature is zero degrees on the Celsius scale, which is pretty much the point where it becomes ice, that is.
The Physical Change of State and the Freezing Point in Celsius
When we talk about freezing, we’re talking about a physical change, not a chemical one. This means the substance itself doesn’t become something new; it just changes its form. Water is still water, whether it’s liquid or solid ice, you see. The molecules, the tiny building blocks of the water, are still water molecules. They haven't rearranged themselves into a totally different kind of molecule. They’ve just changed how they’re moving and how close they are to each other, which is pretty neat. This change happens when the temperature of the liquid drops to that specific freezing point in Celsius, or whatever temperature scale you are using. It’s almost like the molecules decide, “Okay, it’s cold enough now, time to settle down and hold hands,” which they do, more or less.
Every liquid, with the interesting exception of helium under certain conditions, will go through this freezing process when it gets cold enough. Each liquid has its own special temperature where this happens, its own freezing point. For water, that very important number is 0°C. This is the temperature at which water becomes ice, and it’s a number that’s incredibly useful for all sorts of things, from cooking to weather forecasting, as a matter of fact. So, when you hear about temperatures dropping below zero, you know that water is going to start firming up, which is rather significant.
- Mariah Amato Age
- Ashlee Good Funeral
- Jessica Clark Height
- Medley Crossword Clue
- Austin Country Club Wedding
How Does Temperature Affect This Process at the Freezing Point in Celsius?
The temperature of a liquid is basically a measure of how much energy its tiny parts, its molecules, have. When a liquid is warm, its molecules are zipping around with a lot of energy, bumping into each other quite a bit. But as the temperature drops, as the liquid loses heat, these molecules start to slow down. It’s like they’re running out of steam, in a way. This slowing down is key to the whole freezing process, you know. When they reach that specific freezing point in Celsius, they don’t have enough energy to keep moving freely past each other anymore. They start to get sluggish, pretty much.
Molecules Slowing Down at the Freezing Point in Celsius
Once the molecules slow down enough, they start to arrange themselves into a more fixed, organized pattern. They form tighter connections, or "bonds," with their neighbors. Think of it like a bunch of people dancing freely in a room, then the music slows down, and they all start holding hands and standing still in a formation. That’s kind of what happens with molecules when a liquid reaches its freezing point in Celsius and turns into a solid. They stop sliding past each other and instead vibrate in fixed spots. This is why a solid holds its shape, while a liquid flows. It’s all about how much energy those tiny bits have, and how they decide to hang out together, actually.
So, the more heat energy a liquid loses, the colder it gets, and the slower its molecules move. When it hits that particular temperature, that freezing point, the molecules just don't have the get-up-and-go to stay loose anymore. They lock into place, forming what we see as a solid. This is why, for instance, a forecast for freezing temperatures overnight means you might wake up to ice on your car, because the water molecules have lost enough warmth to settle into that firm, unmoving state, which is quite a common occurrence.
What Things Can Influence the Freezing Process and the Freezing Point in Celsius?
While water’s freezing point in Celsius is usually 0°C, it’s not always that simple. There are several things that can actually change when and how a liquid freezes. It’s not just about hitting that exact temperature; other substances mixed in, or even the way the liquid is handled, can make a difference. These influences can either lower the temperature at which something freezes, or change how quickly the freezing happens. It’s a bit like adding different ingredients to a recipe; they can alter the final outcome, you know.
Common Substances That Alter the Freezing Point in Celsius
Think about things like sugar, or even salt. When these are present in water, they can actually make the water need to get even colder before it turns to ice. This is why, for example, salt is put on roads in winter; it lowers the freezing point of water, so ice doesn't form as easily at 0°C. Other things, like muscle tissue in food or even air, can also play a part in how things freeze. Muscle tissue, which contains a lot of water, will freeze, but the presence of other components within it means its freezing behavior might be a little different from pure water. Air, too, can influence the process, sometimes leading to things like freezer burn if not managed well. So, it's not just about the water itself, but what's mixed in with it, that is.
These influences mean that while 0°C is the standard for pure water, you might find liquids freezing at slightly different temperatures depending on what’s dissolved in them. This concept is pretty useful in many areas, from making ice cream (where sugar helps keep it from becoming a solid block of ice) to preserving food. Knowing what factors can change the freezing point is, basically, a helpful bit of information for many everyday situations, as a matter of fact.
Why is Rapid Freezing Often Better for Food When Considering the Freezing Point in Celsius?
When we freeze food, we’re trying to keep it fresh for longer. The goal is to stop tiny organisms from growing and slow down changes that make food spoil. And it turns out, how quickly you freeze something can make a pretty big difference in the quality of the food once it’s thawed. Rapid freezing is often considered the better way to go, and there’s a good reason for that. It’s all about the tiny ice bits that form inside the food, you know.
When food freezes slowly, the water inside it has more time to form larger ice structures. Imagine these as big, jagged crystals. These bigger crystals can actually cause damage to the food’s cells and tissues. Think about a sponge: if you freeze it slowly, the ice forms big chunks that can break the sponge’s structure. When it thaws, it might feel mushy or have a weird texture. The same sort of thing can happen with food, affecting its taste and feel once it’s ready to eat again, which is not ideal, really.
But when food freezes quickly, the water inside doesn’t have much time to gather together and form those big, damaging ice crystals. Instead, it forms many, many tiny ice bits. These smaller ice structures cause much less harm to the food’s natural makeup. The faster the food freezes, the smaller these ice bits tend to be. This means that when you eventually thaw the food, it will keep more of its original texture, flavor, and overall quality. So, for things like frozen vegetables or meats, a quick trip below the freezing point in Celsius, and staying there, is actually pretty important for a good result, basically.
Freezing Rain - A Unique Weather Event Related to the Freezing Point in Celsius
Sometimes, winter weather can be a bit tricky to understand, especially when we talk about things like freezing rain. It’s different from sleet, and it's certainly not just regular cold rain. Freezing rain happens when rain falls through a layer of air that is at or below the freezing point in Celsius, which is 0°C. The rain itself starts as liquid, but then it cools down as it falls through that cold air. However, it doesn't freeze solid until it actually hits a surface that is also at or below the freezing mark, you know.
When freezing rain lands on things like roads, trees, or power lines, it immediately turns into a layer of clear ice. Unlike sleet, which bounces when it hits the ground because it’s already frozen into tiny ice pellets, freezing rain just coats everything it touches. And unlike warm rain, which just runs off surfaces, this cold rain sticks and solidifies. This can make surfaces incredibly slick and dangerous. Because it looks so much like regular rain when it’s falling, people might not realize how dangerous it is until it’s too late. It’s a bit like a sneaky kind of weather, you see, that can catch some folks off guard, which is why it’s important to know about it.
The immediate freezing upon impact is what makes freezing rain so hazardous. It can create very slippery conditions on roads and sidewalks, making driving and walking quite risky. It also adds weight to branches and power lines, which can cause them to break and lead to power outages. So, while the concept of the freezing point in Celsius is simple for water, its manifestation in weather, like freezing rain, can have some rather serious consequences, basically, for daily life.
Understanding Water's Freezing Point in Celsius and Other Scales
When we talk about the freezing point of water, we often use Celsius, and that’s a very common way to measure it around the world. As we've discussed, for pure water, it’s 0°C. But it’s helpful to know that this same temperature can be expressed using other measurement systems as well. For example, in Fahrenheit, water freezes at 32°F, and in Kelvin, it freezes at 273.15 K. These are just different ways of putting a number to the same physical event, you know, the point where liquid water becomes solid ice, which is pretty straightforward.
Knowing these different temperature points can be useful depending on where you are or what kind of information you're looking at. While Celsius is very widely used, especially in science and in many countries for everyday weather, Fahrenheit is common in some places, and Kelvin is typically used in more scientific or technical fields. Regardless of the scale, the core idea remains: it’s the specific temperature where water molecules lose enough energy to stop flowing freely and instead lock into a solid structure. It’s a very important threshold to remember for water, as a matter of fact.
This understanding of the freezing point in Celsius, and how it relates to other scales, helps us grasp how temperature influences the state of matter. It's a fundamental concept that helps us predict weather, preserve food, and even understand how different substances behave when they get very cold. The process, from a liquid turning into a solid because its temperature drops below a certain point, to its molecules slowing down and forming tighter connections, is something that's quite interesting to consider, you know, in various situations.
This article has explored the concept of the freezing point in Celsius, explaining how a liquid changes into a solid by losing heat and how its molecules slow down and form stronger bonds. We've looked at how factors like water, sugar, muscle tissue, and air can influence this process, and why freezing food quickly can improve its quality. We also touched upon the unique phenomenon of freezing rain and how water's freezing point is expressed across different temperature scales like Fahrenheit and Kelvin. It's all about understanding that special temperature where things get firm.
Related Resources:
Detail Author:
- Name : Maurine Conn
- Username : estroman
- Email : carroll.douglas@pagac.com
- Birthdate : 1973-06-29
- Address : 65679 Ayden Cove Heathcotemouth, NY 86710
- Phone : 1-901-963-4876
- Company : Murphy and Sons
- Job : Social and Human Service Assistant
- Bio : Facere nihil cum exercitationem eveniet voluptas magnam. Consequatur et quibusdam est dolorem quia aut consequuntur consequatur. Corporis nostrum sint vero nostrum omnis quos.
Socials
twitter:
- url : https://twitter.com/daisybashirian
- username : daisybashirian
- bio : Laboriosam ab ut nisi fugit et. Tenetur aut occaecati vel quia nulla officiis debitis. Ea quaerat itaque nihil et. Ut qui nulla amet sed quam.
- followers : 5249
- following : 2307
tiktok:
- url : https://tiktok.com/@daisy.bashirian
- username : daisy.bashirian
- bio : Omnis nisi nisi quisquam debitis suscipit.
- followers : 4864
- following : 2887
facebook:
- url : https://facebook.com/daisy7510
- username : daisy7510
- bio : Illo reiciendis deserunt quia ad impedit illum.
- followers : 207
- following : 50
linkedin:
- url : https://linkedin.com/in/bashirian2005
- username : bashirian2005
- bio : Et et sint quia consequatur placeat nostrum esse.
- followers : 4906
- following : 398
instagram:
- url : https://instagram.com/bashirian2006
- username : bashirian2006
- bio : Sed magni vel aut rerum. Incidunt qui voluptatem et nobis eum laudantium qui.
- followers : 2488
- following : 1254